Introduction
Metals are one of the most versatile of all construction materials. The process of refining metals allows them to be constructed into almost any shape with limitless possibilities. Metals in construction are rarely used in their purest form — pure being metals that have not been alloyed (combined) with other metallic elements. Commercially pure metals are 99% pure minimum. Some of the metals listed below are available as commercially pure and many can be manufactured to be extremely pure, often 99.999% minimum, referred to as “five nines min.”
Alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain properties of a metal in the resulting material such as electrical conductivity, ductility, opacity, and luster, but may have properties that differ from those of the pure metals, such as increased strength or hardness. This is typically why alloys are used, in order to add desirable qualities to the metal.
- Example: Adding Chromium and nickel to steel makes the steel corrosion resistant, or stainless. It is the ability to make alloys that makes metals so versatile.
Smelting
The process of refining material to extract the “pure” metal. Once the base metal is obtained, it usually undergoes further treatment to eliminate any impurities that might affect its use.
Ductility
Is a property that allows steel to withstand excessive deformations due to high tensile stresses without failure. The more ductile a metal is, the more it can bend/deform.

Fabricating Metals (forming)
Fabrication refers to the different processes of forming and shaping refined metal into the desired condition. The most basic fabrication method is casting, however there is also rolling, extrusion, etc.
Casting: Molten metal is poured into a form where it is allowed to cool and harden into the desired shape.
Rolling: Process of passing metal through rollers to produce the needed shape. Rolling can be done hot or cold
- Hot: Generally eliminates flaws in the metal. (all metals except iron)
- Most structural steel is hot rolled.
- Cold: Increases the metal’s strength and elastic limit but decreases its ductility. Ductility is the ability of a metal to deform permanently under stress.
- Most smaller structural sections are cold rolled for the increased strength.
- Decreased ductility means the metal won’t deform as much under stress, but instead will fail such as a breakage, shear, etc. The metal will be more ‘rigid’.
Extrusion: Pushes metal through a die to form a shape. Many aluminum sections are formed this way, especially decorative sections and those used for doors and windows. If sufficiently large quantities are needed, then custom shapes and dies can be made for a particular job. Dies can break during the manufacturing process and therefore certain shapes are more difficult to extrude than others.
Drawing: Similar to extruding, but the metal is pulled through a die instead of being pushed through.
- The drawing process usually reduces the size of the piece or changes its shape, but it also improves the strength and surface qualities of the metal. Drawing is applicable for all metals except iron.
Bending: Changes the shapes of tubes and extruded shapes by passing them through various kinds of rolling machines and presses.
Brake Forming: Takes plates and sheets of metal and makes successive one-directional bends to fabricate the shape.
Spinning: Forms round shapes on a lathe.
Embossing: Makes patterns on flat sheets of metal by passing them through a machine with the embossing patterns on rollers.

Treating the Metals
Once the metal is formed, it’s common to treat the metal in different ways to further impart desirable characteristics.
Annealing: The metal is reheated and slowly cooled to obtain a more ductile metal which will have improved its machinability and cold-forming characteristics.
- Meant to relieve stresses in the metal caused by cold working, and can alter ductility, strength, and other mechanical properties.
Quenching: Involves heating the metal (typically steel) to a certain temperature and then rapidly cooling it by complete submersion in water or some other liquid. This strengthens the steel. Quenching makes strong steel, but makes brittle steel. So after quenching, the steel is normally tempered.
Tempering: Similar to quenching but does not involve rapid cooling. It is also used to improve the strength and workability of steel
Case Hardening: Produces a hard-surface steel over a relatively softer core.
- Heating metal and then diffusing a gas (commonly carbon or nitrogen) into its surface, creating a thin layer of a harder alloy.
Finishing Metals
There are three general types of metal finishes. Not all of these finishes are used on all metals. When selecting the type of finish for a metal, considerations of cost, appearance, amount of protection required, long term cost (life-cycle cost) and required maintenance.
Mechanical Finishes: Alter the surface of metal in some way. This can be simply by changing the way the metal comes from the final forming result, or may result from more refined finishing methods such as grinding or buffing.
Chemical Finishes: Are produced by altering the surface of the metal with some type of chemical process. Sometimes this cleans and prepares the surface, other times they protect or color the metal. Anodizing of aluminum is one type where the metal is immersed in an electrolyte bath and a current is applied to the metal. This helps create a variety of colors and also becomes integral to the aluminum structure.
Coatings: Finishes that consist of applied materials such as paint that may be for protection of the metal or purely decorative. Coatings may be clear or opaque.
- Bonderize: Coat with an anticorrosive phosphate solution in preparation for the application of paint, enamel, or lacquer.
| Most Common Polished Finishes For Architectural Work | |
| No 3 | Intermediate, dull finish, coarser than no.4 |
| No 4 | General-purpose polished finish that is dull and prevents mirror reflection. One of the most commonly used. |
| No 6 | Dull satin finish |
| No 7 | Highly reflective polished surface |
| No 8 | Most reflective, used for mirrors and reflectors. Seldom used for architecture (no 7 is used instead). |
Joining Metals
All types of metals can be mechanically fastened using bolts or screws.
- Plastic or rubber washers are needed to prevent Galvanic Action between some metals (see below)
- Small screws can be used to attach light gauge metals, these are typically self tapping
Welding: Joining of two metals by heating them above their melting point. When they cool, the metals physically form one piece of metal.
- Very common for structural steel.
- Not appropriate for thinner sections where the weld will translate through.
Brazing: Joining of two metals at an intermediate temperature by using a non ferrous filler metal with a melting point above 800degreesF (but lower than the welding).
- Usually used for brass, bronze, and some aluminums.
- Results in a clean joint, but some buffing may be required.
Soldering: The joining of two metals using lead-based or tin-based alloy solder filler metal that melts below 500 degrees F.
Adhesives
Metals can also be fastened with adhesives. This method is usually reserved for small trim pieces and sheet stock where the strength of the bond is not critical. Normally, adhesives are used when fastening metals to other substrates such as plywood and particle board.

Electrolysis (Galvanic Action)
Galvanic Action is the corrosion that happens when dissimilar metals come in contract with each other in the presence of an electrolyte… most commonly moisture in the form of rain or condensation. This creates a mild electrical current which gradually will corrode one of the metals while the other will remain intact.
- In short, two dissimilar metals in contact with each other will cause a reaction which may lead to corrosion.
- The way to solve this is to keep reactive materials separate via some means. This is typically done with mastic, building paper, neoprene, plastic, rubber, or other non-conductive material.
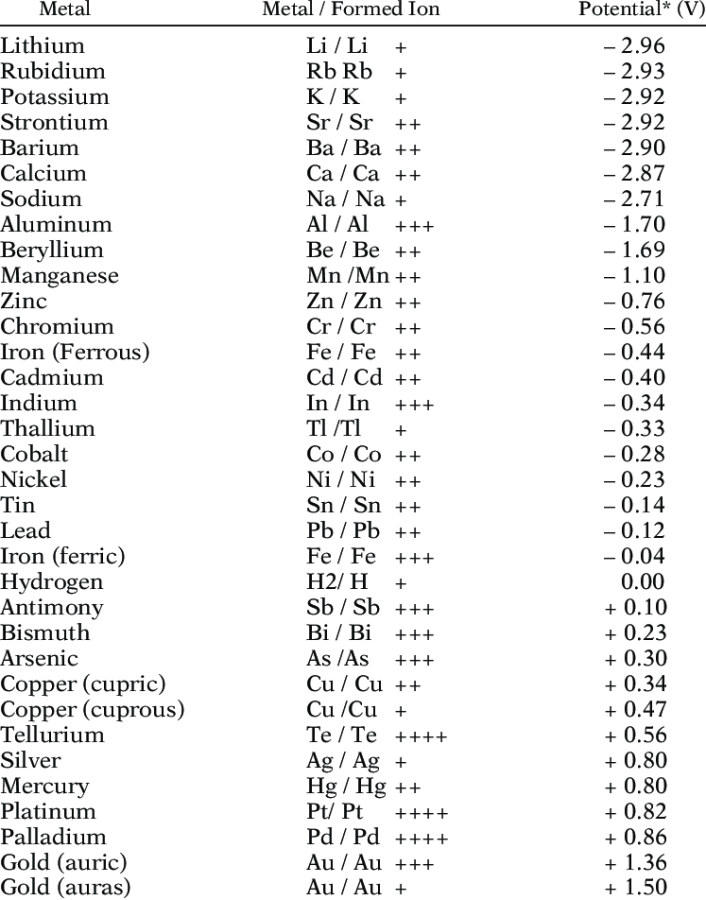
Galvanic Scale
To understand ‘how’ dissimilar materials are, the galvanic scale may be used. The further away the materials are on the scale, the greater the possibility is of corrosion when they come in contact. If the difference is negligible, there is low likelihood that galvanic action will occur. Therefore, to reduce the possibility, you should either use similar metals or separate the metals with a non-conductive surface as discussed earlier.
- Electrolysis is most severe in humid and marine environments, where there is an abundance of seawater. It is much less of a concern in arid dry climates.
Metal Nobility
Nobility in metals refers to a metal’s resistance to corrosion and oxidation, and its lack of chemical reactivity. Noble metals are generally scarcer and more valuable than base metals. They are often found in nature in their raw form.
Ferrous and Non-Ferrous Metals
There are two major classifications of metals, ferrous and non-ferrous.
Ferrous Metals:
Those that contain a substantial amount of Iron.
- Typically also contain some amount of Carbon
- The amount of carbon, and other elements added to the Iron determines the strength, ductility, and other properties of the ferrous metals.
- Ferrous Examples: Iron, Steel, Stainless Steel
- All ferrous materials will rust or corrode unless protected.
Non-Ferrous Metals:
Non-ferrous metals are metals that contain no iron. By having no iron, they are more resistant to corrosion and rust than ferrous metals. They are also non-magnetic, which makes them useful for certain applications such as electronic and wiring applications.
Non-Ferrous Metal Examples:
- Aluminum, Copper, and Copper Alloys (Bronze & Brass), Zinc, Lead, Gold
Aluminum
Aluminum by itself is soft and weak, however, alloying it with other materials improves strength and hardness. One of the major disadvantages with aluminum is its high amount of energy required to refine & produce the product. The material is recyclable, but requires a lot of embodied energy.
Aluminum has been used in the construction industry since the 1900s. It’s a versatile metal with many advantages, including:
- Lightweight
- Corrosion resistance
- Electrical and thermal conductivity
- Reflectivity and ductility
- Odorless and impermeable
- Recyclability
- Strong and durable
- (technically) Infinitely recyclable
Uses in Architecture:
- Roofs and walls (exterior & interior)
- Windows and doors (mullions/jambs/etc)
- Staircases (railings and stairs)
- Cladding & Flashing
- Mechanical systems such as air conditioning
- Heating systems and components
Mechanical Finishes
There are lots of different finishes and textures available for aluminum. Buffed, directional textured, non-directional textured, patterned, and chemical are all options that provide a variety of aesthetics.
- Chemical finishing is normally an intermediate step into preparing it for another type of finish.
Joining Aluminum
Screwing, bolting, welding, brazing, soldering, and adhesive are all available for aluminum.
- Adhesive should only be used in limited situations where high strength is not needed. It’s always best to mechanically fasten pieces together.
Wrought Iron and Cast Iron
Wrought Iron
Iron with a very low carbon content (less than .30%) and a substantial amount of slag. Similar in chemical composition to low-carbon steel, but most of the impurities are in the slag which is mechanically mixed with the iron.
- Soft, ductile, and resistant to corrosion.
- Use is limited to ornamental iron work such as gates, grilles, and fences.
Cast Iron
Iron with a carbon content above 2%. With the higher percentage of carbon, it’s very hard but brittle.
- In the nineteenth century it was used for columns and beans
- Cast Iron with a low silicon content is called white cast iron and has little use in construction unless it is processed in such a way as to produce malleable iron.
- Cast iron with a high silicon content is called gray iron and is used for types of castings such as plumbing valves, pipes, and hardware.
Steel
Steel is one of the most widely used because of its many advantages, which include high strength, ductility, uniformity of manufacture, variety of shapes and sizes, and ease of speed of erection. Steel is used in a variety of structural and nonstructural applications including columns, beams, concrete reinforcement, fasteners, curtain wall panels, pipes, flashing, etc.
- Ductility of steel makes it very useful for earthquake resistant structures.
- Steel is manufactured under controlled conditions so its composition, size, and strength can be uniformly predicted. Steel structures therefore do not need to be greatly oversized (as concrete or wood structures) in order to compensate for manufacturing or erection variables.
Disadvantages of steel:
Steel is not good with fires. Steel has great reduction in strength when subjected to fire and its tendency to corrode in the presence of moisture. Steel does not burn, but instead deforms when exposed to high temperatures.
- Therefore, steel must be protected with fireproofing.
- Steel will rust and corrode if not protected from fire and moisture. This can be prevented by including other elements into the steel to make it corrosion resistant (such as stainless steel), or by covering the steel with a protective coat of paint or other finish/coating like an elastomeric coating.
The percentage of carbon in steel directly affects its strength and ductility of steel. As carbon is added, the strength increases but the ductility decreases.
- Standard structural steel has .20 to .50% CARBON
Low-Carbon Steel: contains .06% to .30% Carbon
Medium-Carbon Steel: Contains .30 to .50% Carbon
High-Carbon Steel: Contains .50 to .80% Carbon.
Stainless Steel
Stainless Steel is a metal alloy containing a minimum of 11% chromium. In addition nickel is often added to increase corrosion resistance and improve cold workability. Stainless steel is available in a variety of shapes and forms, including sheets, wires, bars, and plates. Lots of structural shapes such as T and H sections are also available.
- Highly corrosion resistant and stronger than other architectural metals. This is due to the formation of a chromium-oxide film on the surface of the metal. If the film is scratched or otherwise damaged, it will reform as the metal is exposed to oxygen in the air (this is called passivity).
- Passivity: A layer of nonreactive molecules that does not allow metal ions at the surface to migrate into the solution. A metal with passivity is considered a passive metal.
- Most stainless steels remain passive, however, when the layer is lost to abrasion or chemical etching that introduces chlorides or free irons, the surface can become active again. This affects the corrosion resistance as well as the portion of the steel in the galvanic series.
- There are about 40 types of stainless steel. Only 8 are used for construction: 6 for products and 2 for fasteners.
- There are also patterned finishes available that are produced by passing the sheet between patterned rollers. Color coatings are also available.
| STAINLESS STEEL TYPE | DEFINITION | TYPICAL USE |
| TYPE 301 | Similar to Type 302. Smaller amounts of chromium and nickel. It is still very corrosion resistant. Improved work-hardening properties which can result in high tensile strengths | Structural Applications |
| TYPE 302 | Contains 18% chromium and 8% nickel. One of the most widely used stainless steel types. Highly resistant, very strong and hard, and can be easily fabricated by standard techniques. | Commonly Used. |
| TYPE 304 | Largely replaced by Type 302. 302 is easier to weld and the properties are nearly the same. | n/a |
| TYPE 316 | For extreme corrosive environments. | Industrial plants, marine locations |
| TYPE 430 | Does not contain any nickel, less corrosion resistant than the others. | Interior applications |
Other Alloy Steels
Various elements can be added to steel to impart certain qualities. The fact that steel and metals can be combined with others to add certain characteristics is what makes metals so valuable in the first place.
- ASTM (American Society of Testing and Materials): Tests lots of different materials and designates and classifies them.
- ASTM A36: Most common type of structural steel.
- ASTM A440: High strength structural steel used for bolted or riveted connections.
Weathering Steel: Alloy that contains a small amount of copper. When exposed to moisture in air or from rain, it develops a protective oxide coating with a distinctive sepia-colored finish. It is used where it is difficult to maintain the steel, or it is used for appearance. Because of the oxide that is carried off by rain, it should be detailed so that it does not stain other materials.
Copper and Copper Alloys
Copper is widely used because of its resistance to corrosion, workability, and high electrical conductivity.
- Copper is used for electrical wire primarily due to its high electrical conductivity.
- It’s also used for hardware, curtain walls, piping, gutters, and other ornamental purposes.
Structural Shapes
Below are listed more common structural member shapes. There are many more besides this chart such as miscellaneous shapes and others.
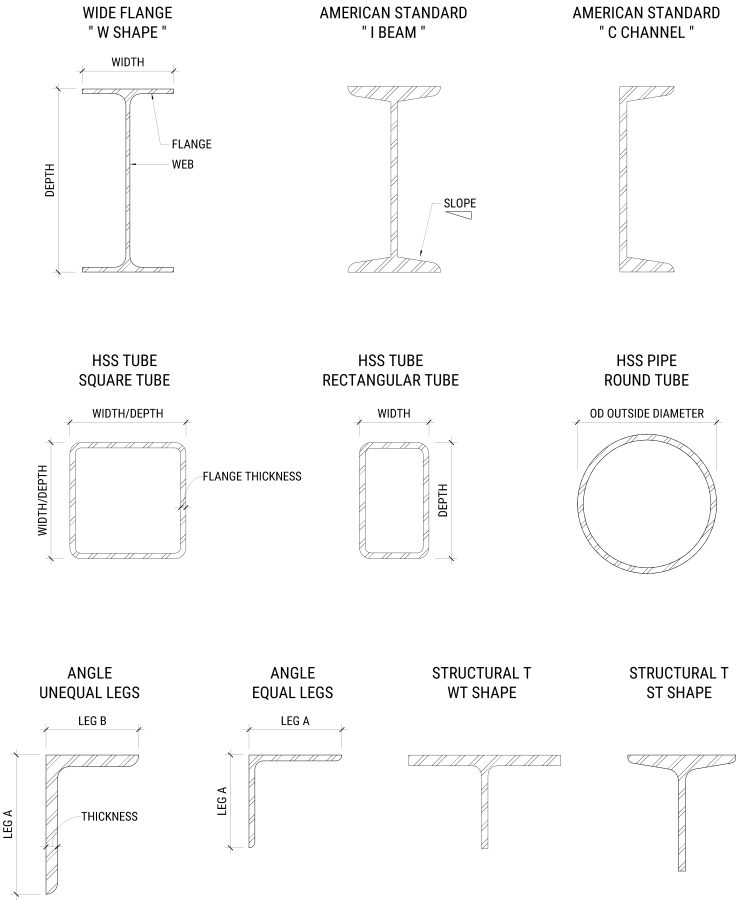
Below are listed more common structural member shapes. There are many more besides this chart such as miscellaneous shapes and others.
| NAME | NICKNAME | DESCRIPTION | EXAMPLE |
| Wide-flange members | W-shapes | Called Wide-flange because the width is wider than that of standard I beam shapes. Designated with the “W” followed by nominal depth (inches) and the weight in lb/ft.Commonly referred to (incorrectly) as I beams.NOTE: Actual depth varies a little based on the manufacturing process. | W18x85= 18in deep and 85 lbs/ft. |
| American standard I-Beam | I beams | Narrow flange and includes a slope on the inside face.Designed with “S”Typically used for beams only. | S15x50= 15in deep, and 50 lbs/ft |
| American standard Channel Section | C Channel | Designated with the letter “C” | C6x13= 6in deep, and 13 lbs/ft |
| Structural Tees | T Shapes | Generally made from cutting a W-Shape or I-Beam in half.Cut from a W-shape = WT designationCut from an I-Beam = ST | WT9x57 came from a W18x114ST7.5×25 came from an S15x50 |
| Steel Angles | L shaped angles | Available with equal or unequal legs. Designated by the letter L. Followed by the lengths of the angles and then followed by the thickness of the legs.LLV and LLH are sometimes used to indicate orientation if not clear from detailsLLV = Long Leg VerticalLLH = Long Leg Horizontal | L6 x 4 x ⅜= 6” Leg with 4” leg and ⅜” Thickness (unequal legs) L4 x 4 x ¼”= 4” Leg with 4” leg and ¼” thick |
| Square And Rectangular Tube Sections | HSS Pipes | Structural tubing and Structural Pipe is available in a multitude of standard shapes and sizes. Used typically for lighter framing or members of a larger truss. Sometimes where aesthetics are a primary concern.Round HSS tubes have decimal numbers, while square have rational integer numbers. | HSS5.563 x 0.258= 5.563” OD of a tube with .258” wall thickness HSS4x8x.25 = 4” x 8” tube with .25” thickness |
| Bars | Bars are any rectangular section 6” or less in width with a thickness of .203 inches or greaterOr Sections 6-8” wide with a thickness of .230 inches and greater | .25”x4”=.25” bar thickness by 4” wide | |
| Plates | Any section over 8” wide with a thickness of .230 inches and over.Or Sections 48” wide with a thickness of .180” and over. | PL 1/2” x 12”=.50” plate thickness x 12” wide |
Gauge & Thickness
The thickness of different metal products (sheet steel, tubing, strips, etc) is normally expressed as a gauge, or thickness in either decimal inches or decimal mm.
Gage numbers come from olden times when metal was sold based on weight, however, this was not efficient since density, coatings, etc. all affect the metals and are not reflected in the gauge. Different manufacturers also had their own gauge numbers, which might still be used today.
Because of this, it’s always better to call out and specify the actual desired thickness in decimals of inches or mm depending on imperial or metric systems used. Also note that gauge #7 in brass, will not be the same as gauge #7 in copper — there will be a different definition from materials to material.
Variations
Always reference the manufacturers pricing tables/gauge charts/etc for exactly what is defined as what. Remember that due to the manufacturing process, variations in thickness are to be expected.
Design Notes: Metals Expansion and Contraction
Metals expand with the changes in temperature more than other materials, so it’s important to allow for the metal to expand and contract with the detailing by using slip joints or other types of similar jointing. Its common in structures to have one side ‘pinned’ while the other is floating for expansion.
Other Thoughts
- Bolts are relatively inexpensive and easy to install.
- Welded studs – minimize the number of holes to punch for bolts
- Welding is the best for moment connections
- Rivets used to be popular, but are seldom used now. Their cost is usually higher than bolted or welded
Specialty Metals
There are limitless options of what you can do with metals and types that can be developed. Listed below are a couple specific cases that are more commonly used but still rare.
Monel Metal
Used primarily for roofing, a combination of copper and nickel with small amounts of other elements. Highly resistant to corrosion and is easily worked. It also has good fatigue resistance and high resistance to cracking.
Zinc
Resistant to corrosion and is sometimes used for sheet roofing and flashing. Zinc fasteners are also made. The metal is more commonly used for coating steel to produce galvanized steel.
- Zinc strips can be used in roofing to slightly electrify the way and prevent algae build up on a roof. Typically one strip is installed near the ridge.
Lead
Lead is also resistant to corrosion and is occasionally used for complex roof shapes. However, its density makes it ideal for acoustical insulation, vibration control, and radiation shielding. Its common use now is in hospital and other specialty environments where shielding is needed in the walls for equipment or other electromagnetic singaling.
Template
An alloy of 75% lead and 25% tin can be used to plate steel for roofing. Common uses are downspouts, gutters and similar.
Leave a Reply
You must be logged in to post a comment.